Soil Science 100
Final Exam 2 ID____________________
Friday 17 December 1999 Lab Section___________________
Value 150 points
Answer all questions in the space provided.
1. (15) Heterotrophic bacteria utilize preformed carbon sources for energy. How does their use of these carbon sources affect soil pH, composition of the soil atmosphere, and availability of nitrogen to plants? For complete credit, you must answer each part of this question by indicting how each component changes (e.g. up down, more less) and the mechanism or process that produces the change.
The pH is decreased as carbon in organic matter is oxidized to carbon dioxide.
Respiration depletes oxygen and increases carbon dioxide in the soil atmosphere.
Assimilation of carbon requires the mineralization and assimilation of some nitrogen. As the organisms die, the nitrogen is released, thus increasing the N supply to plants.
2. (10) Name each of the five factors of soil formation enunciated by Jenny (exclude the human factor).
Parent material, organisms (biota), topography, climate and time.
3. (20) Draw a diagram showing five components of the nitrogen cycle. For each component in the diagram use arrows to show transfers among the components. Label each arrow with the name of the process and the form of nitrogen.

4. (10) What are two differences in the reclamation of saline soil and sodic soil? What are the similarities in reclamation of these two problems?
The two main differences are the need for acid or acid producing compounds to lower the pH and cations other than sodium to replace sodium on the exchange complex when reclaiming sodic soils compared to those that are saline. Similarites are that good drainage (artificial or natural) and sufficient water for leaching are required by both reclamation processes.
5. (10) Write the Darcy equation for saturated flow in soils and define each of the variables in the equation.
Q = K*A*DH/DX or d
Y/dXQ= flow rate, K= hydraulic conductivity or constant representing ease of flow,
DH/DX or dY/dx the driving force, change in head or potential per length.6. (15) Explain the results of the fertilizer experiment that produced the following graph. Your explanation must include statements about each of the four parts of the curve.
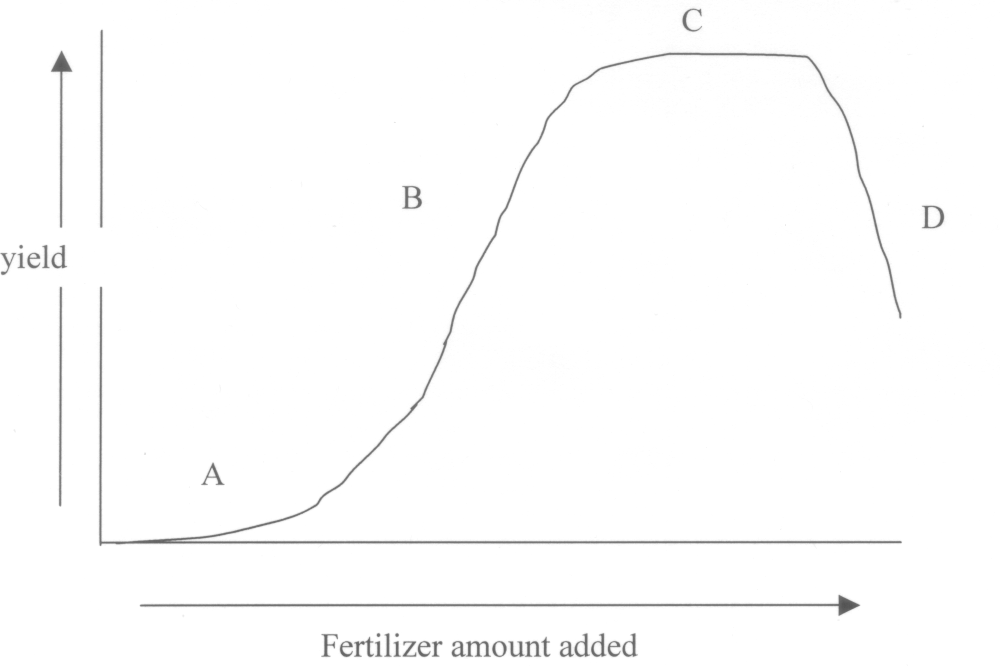
Region A fertilizer amount is too low to stimulate significant response from the crop.
Region B, yield increases rapidly as the fertilizer amount increases because the deficiency is being reduced.
Region C, yield plateau as the fertilizer is in sufficient quantity for meeting the plants genetic potential
Region D, Excess fertilizer leads to a toxicity which causes yield to decline.
7. (10) List one advantage and one disadvantage of the following kinds of diagnostic tests for diagnosing plant nutrient deficiencies: Soil tests, leaf tissue tests, field plot trials, greenhouse pot trials, plant deficiency symptoms.
|
Test |
Advantage |
Disadvantage |
|
Soil test |
quick, easy ?, relatively inexpensive, quantitative |
sometimes expensive, timing is important |
|
Leaf tissue test |
quick, easy, relatively inexpensive, quantitative |
sometimes expensive, timing is important (too late to respond) |
|
Field plot trials |
quantitative |
long term, slow, expensive, timing |
|
Greenhouse pot trials |
quantitative, controlled |
slow, expensive, timing |
|
Deficiency symptoms |
direct, cheap |
timing, qualitative, difficult to interpret |
8. (30) Listed below are five geomorphic positions you would find in an east to west transect from the Sierra Nevada to the Pacific Coast. For each position, complete the table. You are not expected to know the exact value of these variables, but you should be able to fill in each box so that the relative value of variables is consistent. If you think that a horizon does not exist at a location, fill in the space with the word missing or absent.
|
Position |
A horizon color |
A horizon pH |
A horizon thickness (cm) |
Bt horizon thickness (cm) |
Bt horizon color |
Bt horizon pH |
|
Mid-elevation upland on granitic bedrock |
Pale, light color, gray |
Acidic |
Thin to moderately thick |
Thick |
Red |
acidic |
|
Old alluvial fan on granitic alluvium |
Brown |
Acidic |
Thin to moderately thick |
Thick |
Red |
acidic |
|
Basin rim
|
Almost any color |
High |
Almost any |
Absent to thick |
Grey or dark, maybe yellow |
High |
|
Low elevation upland on sedimentary bedrock |
Brown |
Neutral to slightly acid |
Thin to moderately thick |
Absent to thin |
Light red to brown |
Neutral to slightly acid |
|
Serpentinite
|
Dark, reddish-black |
Neutral to basic |
Moderately thick to thick |
Moderately thick |
Dark red |
Neutral to basic |
9. (15) List three differences between inorganic fertilizer and organic fertilizer materials. For each, explain how the difference would influence your use of the material in your home garden.
|
Organic |
Inorganic |
|
Low analysis |
High analysis |
|
High variability |
Low variability |
|
Need lots |
Need less |
|
Cheep (sometimes) |
Expensive (sometimes) |
|
More difficult to apply |
Easier to apply |
10. (15) Complete the following table. For each condition,what surface horizon pH (acid, neutral or alkaline) do you expect to find? How (e.g. what process) does the condition contribute to the soil pH?
NOTE: Each condition must be considered separately. These are not conditions found for any single soil or location.
|
Condition |
pH |
Process |
|
The soil forms under mixed deciduous forest |
Neutral to slightly acidic |
Accumulation of base-rich litter, decomposition leading to release of protons, balanced by nutrient cycling of bases. |
|
Precipitation commonly exceeds evapotranspiration by a factor of 3 or 4 on limestone parent material. |
Acid |
Leaching, removing bases and substituting protons for bases on the exchange complex |
|
Soil is flooded continuously during the summer. |
Alkaline/neutral |
Reduction consumes protons raising the soil pH |
|
Soils are heavily fertilized with animal manure. |
Acid |
Mineralization of N releases protons. Also organic acids generated from organic matter additions lowers pH. |
|
Free water (a water table) is found at 1 meter below the surface in a semi-arid climate zone. |
Alkaline |
Evaporation leaves behind salts and sodium raising the pH. |