Soil Science 100 ID__
_KEY Second Exam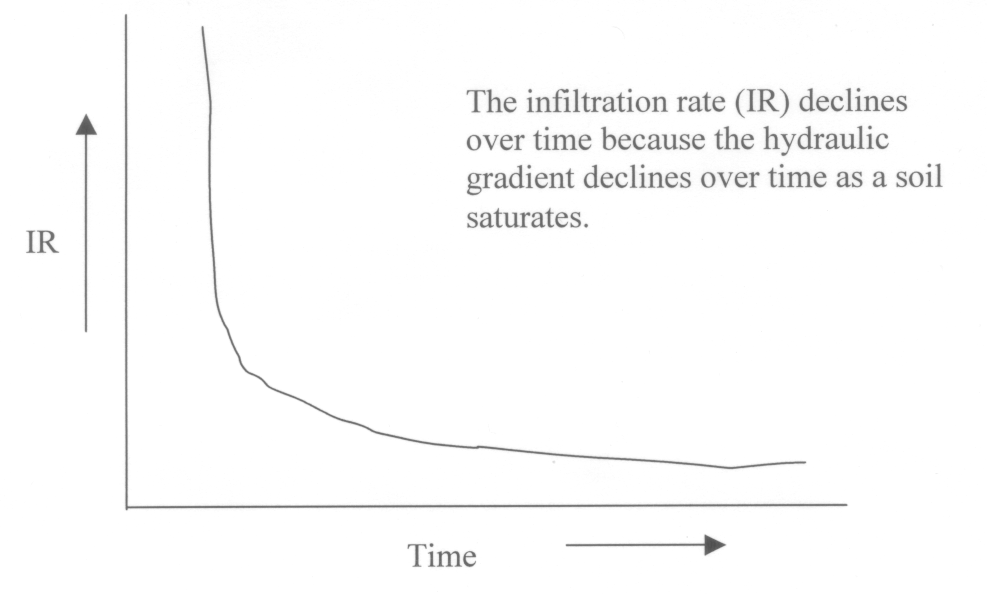
5. (15) a. What is a mycorrhizal association? b. Name one way such associations benefit each of the participants?
A symbiotic association between fungi and plant roots.
The fungus gets readily assimilated carbohydrates from the plant. The plant may get immobile nutrients like P, water, and protection from predators or disease from the fungus.
6. (12) Complete the following table by calculating either the mass of the element needed for the number of cmolc or the cmolc represented by the mass of the atom. Note the atomic mass/mole of Mg = 24, Al= 27, K=39, and Ca= 40.
3 points each correct answer.
|
Ion |
Mass (g) |
cmolc |
|
Mg2+ |
0.72 |
6 |
|
Al3+ |
0.81 |
9 |
|
K+ |
1.56 |
4 |
|
Ca2+ |
0.80 |
4 |
Mg has 0.14 g/cmolc * 6 cmolc = 0.72 g, Al has 3 cmolc in 0.27 g = 0.81/.027=3*3=9
K has 1 cmolc/.39g. 1.56/.39 = 4; Ca has 1 cmolc/0.20g. 0.80/0.20 = 4.
7. a. (15) List 3 reasons why cation exchange is significant to plants, water or people.
CEC allows soil to hold nutrients and toxins making nutrients available to plants, keeping toxins and nutrients out of water supplies.
Readily available source of nutrients
Protects the soil from leaching losses of cations
Potential for renovation of wastes
A pH and nutrient buffer
8. (10) The following figure is from chapter 5 of the text. Use it to answer the following question. Can the aquifer illustrated here supply water to an alfalfa field that requires a volume of 235 m3/day? Assume the pressure heads do not change during pumping. The aquifer has a cross-sectional area of 15,000 m2 and a hydraulic conductivity of 0.75 m/day. In addition to answering yes or no, for full credit, .show the calculations used to determine your answer.
This is a Darcy's law problem. Q = K*A* dH/dX
dH = (125 + 210) - 90 = 245m
dX = 9,999m
A = 15,000 m2
K = 0.75 m/day
Q = 0.75 * 15,000 * 245/9999 = 275.6 m3/day.
YES, the aquifer can supply the needed volume of water each day.